
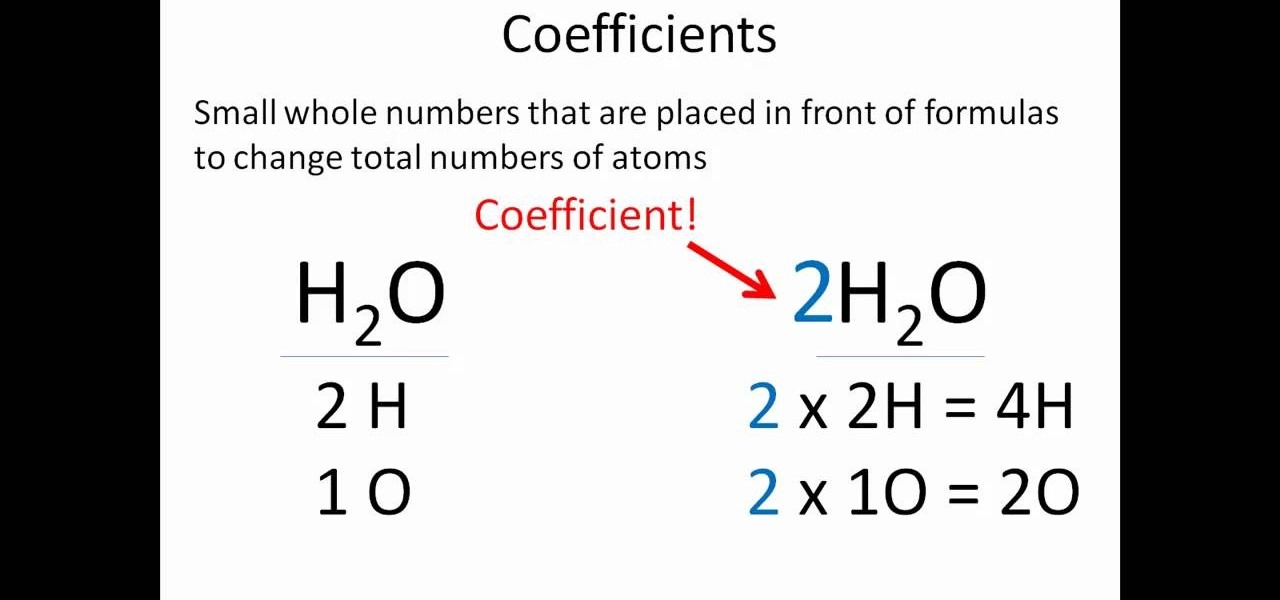
For a more complicated example that has more than one element besides Oxygen or Hydrogen, pick the element that has more atoms on one side compared to the other.Ģ. Leave hydrogen and oxygen for last because they are more often found in more than one chemical on each side of the equation.ī. Pick an element that appears in one molecule on the left side and in one molecule on the left.Ī. Symbols are used to differentiate between different types of reactions.1. The stoichiometric coefficients (the numbers in front of the chemical formulas) result from the law of conservation of mass and the law of conservation of charge (see "Balancing chemical equations" section below for more information). It also indicates that two sodium molecules are required for every two hydrochloric acid molecules and the reaction will form two sodium chloride molecules and one diatomic molecule of hydrogen gas molecule for every two hydrochloric acid and two sodium molecules that react. This equation indicates that sodium and HCl react to form NaCl and H 2. Using IUPAC nomenclature, this equation would be read as "hydrochloric acid plus sodium yields sodium chloride and hydrogen gas."

This equation would be read as "two HCl plus two Na yields two NaCl and H two." But, for equations involving complex chemicals, rather than reading the letter and its subscript, the chemical formulas are read using IUPAC nomenclature.

The two are separated by an arrow symbol (→, usually read as "yields") and each individual substance's chemical formula is separated from others by a plus sign.Īs an example, the equation for the reaction of hydrochloric acid with sodium can be denoted: A chemical equation consists of the chemical formulas of the reactants (the starting substances) and the chemical formula of the products (substances formed in the chemical reaction).


 0 kommentar(er)
0 kommentar(er)
